CONTRACT DEVELOPMENT
The Incipio Devices contract development model is to assemble a core team of experienced design and development resources focused on the orthopedic space.
The core team approach allows Incipio Devices to provide a self-sufficient group resource that internally manages personnel and skill sets to most effectively deliver on stated objectives. Each member of the team has the background and talent to drive all aspects of product development and launch. For any given project, Incipio Devices will leverage the combined resources of the core team to support the project needs.
CAPABILITIES:
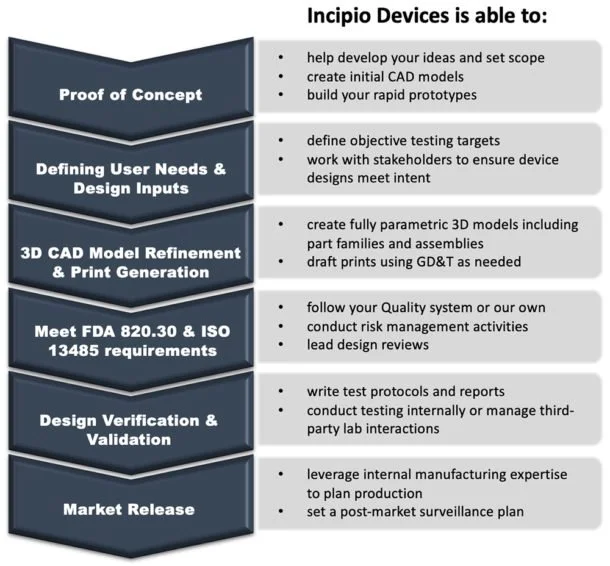
Design and development of medical devices from concept through manufacturing and release.
All elements of design assurance within a variety of quality systems; internal quality assurance resources.
3D modeling, component, and assembly drawings; manufacturing prints and models.
FEA and mechanical development/testing; statistical and functional relationship analyses.
Regulatory clearance, including 510(k) and CE marking.
Internal manufacturing resources for prototyping, design for manufacture assistance, and final production of implants/instruments.
Project partnership directly with surgeons, entrepreneurs, and small to large orthopedic companies.
ISO 13485:2016 and CE certified.
EXPERIENCE:
Development and CAD personnel with 69 years of combined experience in medical device design/development; hundreds of devices taken from concept to clearance and sold nationally/internationally.
All Development personnel have previous experience with large and small orthopedic OEM companies and are familiar with various procedures and systems.
Personnel have experience in solutions for hips, knees, extremities, trauma, spine, and sports medicine; also experienced in project management and OEM remediation activities.